About Us
We would encourage you to download a copy of our Molecular Modeling Guide which will walk you though framework model building with DARLING MODELSTM. Visit DARLING MODELSTM in Use to see how to expand your use of all our models. Contact us when you have questions or need suggestions on the use of our models.
We are Unique.
 Unlike
many other molecular modeling kits, MOLECULAR VISIONSTM design
enables the user to create atom models with as little as two parts. Only
8 unique parts are used to create atoms "with bonds" which in
turn may be joined into an infinite variety of molecules.
Unlike
many other molecular modeling kits, MOLECULAR VISIONSTM design
enables the user to create atom models with as little as two parts. Only
8 unique parts are used to create atoms "with bonds" which in
turn may be joined into an infinite variety of molecules.
sp3 piece, sp2 piece, Double-bond-pi piece, Half-double-bond-pi-Piece, Triply-bonded-atom pair
 Linear
bond, Trigonal planar pieces (2 rods & 1 tube, 1 rod and 2 tubes), Octahedral
&1 tube, 1 rod & 2 tubes)
Linear
bond, Trigonal planar pieces (2 rods & 1 tube, 1 rod and 2 tubes), Octahedral
&1 tube, 1 rod & 2 tubes)(The cube on the pi bond is for pi bonding)
 With
4 auxiliary pi bonding pieces, organometallic models may be represented
as well as transition states.
With
4 auxiliary pi bonding pieces, organometallic models may be represented
as well as transition states.
Bonding pieces used for coordination
Our
solid-state models consist of 4 cation types and 2 anion types. Most lattices
require only one type of anion and one type of cation for the entire model
thus simplifying construction. The cations have the rods and anions have
the tubes. This facilitates the building of crystals from packing diagrams
without special instructions or spacers, in the same manner that the organic
and inorganic molecules are constructed. The limited number of tubes on
the anion simplify cation attachment. The general anion has tubes for only
tetrahedral cation or only octahedral cation insertion. The hexagonal anion
is only used with the octahedral cation.
 The
Anions, general anion, hexagonal anion.
The
Anions, general anion, hexagonal anion.
The Cations, tetrahedral, octahedral, 8 coordinate, tetrahedral anion-cation
Framework
Models
 A
tetrahedral atom with bonds is prepared by joining two sp3 pieces as shown.
- sp3 assembly and final click.
A
tetrahedral atom with bonds is prepared by joining two sp3 pieces as shown.
- sp3 assembly and final click.
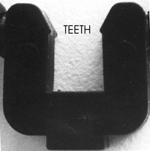 This
reduces the number of pieces required to form molecules. It is important
to note that the center "U" piece contains a pair of "TEETH"
that must be engaged with the "SQUARE" at the end of the other
piece to fully lock the pieces together.
This
reduces the number of pieces required to form molecules. It is important
to note that the center "U" piece contains a pair of "TEETH"
that must be engaged with the "SQUARE" at the end of the other
piece to fully lock the pieces together.
 Form
bonds by inserting a rod from one atom into the tube of another atom. A
compressed ring on the rod locks it inside the tube providing integrity
to a model and free rotation for single bonds.
Form
bonds by inserting a rod from one atom into the tube of another atom. A
compressed ring on the rod locks it inside the tube providing integrity
to a model and free rotation for single bonds.
 The
other atom centers "with bonds" are made in like fashion by joining
the two pieces to engage the teeth.
The
other atom centers "with bonds" are made in like fashion by joining
the two pieces to engage the teeth.
 Space
filling atoms may be formed from the framework piece by adding our new ATOM
VISIONSTM balls. The "AV" after a kit name indicates
that these balls are included.
Space
filling atoms may be formed from the framework piece by adding our new ATOM
VISIONSTM balls. The "AV" after a kit name indicates
that these balls are included.
Other models enhanced by the ATOM VISIONTM balls may be viewed at our section, DARLING MODELSTM in Use.

The addition of the ATOM VISIONTM ball to the triply bonded atoms easily differentiates between the atoms of an alkyne, nitrile and carbon monoxide.
Other models enhanced by the ATOM VISIONTM balls may be viewed at our section, DARLING MODELSTM in Use.
 Those
wishing nearly exact angles on some of the atom pieces may modify any of
the framework models by immersing the piece in boiling water for a minute
or more. The piece is modified and placed under cold water to fix the new
angle. Be sure to clamp or wire the center "U" so that it does
not open or close during the procedure. Examples of this technique are shown
in the distortion of the triple bond in a cobalt complex or the 105 degree
angle of a square pyramid.
Those
wishing nearly exact angles on some of the atom pieces may modify any of
the framework models by immersing the piece in boiling water for a minute
or more. The piece is modified and placed under cold water to fix the new
angle. Be sure to clamp or wire the center "U" so that it does
not open or close during the procedure. Examples of this technique are shown
in the distortion of the triple bond in a cobalt complex or the 105 degree
angle of a square pyramid.
 Of
course after the atom "with bonds" has been formed you may wish
to take it apart. This is done with a very gentle opening of the central
"U" to release the teeth. For example to unlock the tetrahedral
atom the thumbs slightly spread the lower sp3 piece while the finger pushes
the other sp3 piece through. This can eventually be done with one hand.
Of
course after the atom "with bonds" has been formed you may wish
to take it apart. This is done with a very gentle opening of the central
"U" to release the teeth. For example to unlock the tetrahedral
atom the thumbs slightly spread the lower sp3 piece while the finger pushes
the other sp3 piece through. This can eventually be done with one hand.
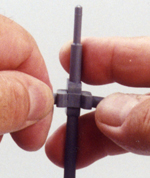 For
each of the other atoms there is a preferred disassembly. The trigonal bipyramid
requires a gentle spreading of the planar trigonal piece while pushing the
linear piece out.
For
each of the other atoms there is a preferred disassembly. The trigonal bipyramid
requires a gentle spreading of the planar trigonal piece while pushing the
linear piece out.
 The
octahedral atom disassembly involves the simultaneous opening of both pieces
The
octahedral atom disassembly involves the simultaneous opening of both pieces
Solid State
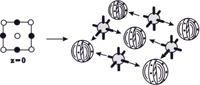 The
crystal unit cells of ionic materials are formed by the attraction of
anions and cations. Before constructing a model you need to know the symmetry
of the anions and cations and whether the packing is cubic or hexagonal.
Usually a packing diagram has been produced from an x-ray structure of
the substance so this model may be followed.
The
crystal unit cells of ionic materials are formed by the attraction of
anions and cations. Before constructing a model you need to know the symmetry
of the anions and cations and whether the packing is cubic or hexagonal.
Usually a packing diagram has been produced from an x-ray structure of
the substance so this model may be followed.
-- A sodium chloride crystal layer from the packing diagram of a cubic face centered cell with octahedral cations.
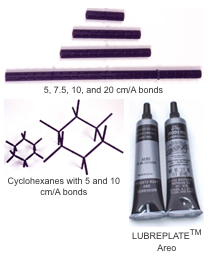 Demonstration
The
demonstration models are made differently from the student models to facilitate
molding. There are atom centers which have rods and bonds 0.5 inches in
diameter which have tubes. The size of the model is determined by the length
of the bonding piece. The pieces are made of nylon and are more rigid than
the student polypropylene models but may still be deformed to produce small
rings. A lubricant, LUBRIPLATE®, is recommended to ease insertion of
the rod, with the compressed ring, into the tube. Check the DARLING
MODELSTM in Use section for suggestions or e-mail us for
help.
Demonstration
The
demonstration models are made differently from the student models to facilitate
molding. There are atom centers which have rods and bonds 0.5 inches in
diameter which have tubes. The size of the model is determined by the length
of the bonding piece. The pieces are made of nylon and are more rigid than
the student polypropylene models but may still be deformed to produce small
rings. A lubricant, LUBRIPLATE®, is recommended to ease insertion of
the rod, with the compressed ring, into the tube. Check the DARLING
MODELSTM in Use section for suggestions or e-mail us for
help.
Caring for the Molecular VisionsTM Pieces.
The material used for the manufacture of the student framework models is a thermoplastic polypropylene copolymer. It will bend when sufficient pressure is applied. Treatment with boiling water for about one minute will return the pieces to their original angles, restore the dimensions of pieces that were cold formed, and remove distortions in pieces which were kept for an extended period in models of strained molecules.
The pieces should be grasped firmly when pushing the rod into the tube so as not to bend or shear the rod piece. Occasionally the joining offers more friction than desired. This may be alleviated with a light spray of silicone or a thin film of LUBRIPLATE® lubricant. Oil lubricants do not seem to work as well.